 |
Origin of high-Mg melts by volatile fluxing without significant excess of temperature |
Alexei V. Ivanov1, Samuel B. Mukasa2, Vadim S. Kamenetsky3, Michael Ackerson4, Elena I. Demonterova1, Boris G. Pokrovsky5, Nikolay V. Vladykin6, Maria V. Kolesnichenko7,8, Konstantin D. Litasov7,8, Dmitry A. Zedgenizov7,8,9
1Institute of the Earth’s Crust, Siberian Branch of the Russian Academy of Sciences, 128 Lermontov street, 664033 Irkutsk, Russia; aivanov@crust.irk.ru ; dem@crust.irk.ru
2Department of Earth Sciences, University of Minnesota, 116 Church Street SE Minneapolis, MN 55455, USA; mukasa@umn.edu
3School of Physical Sciences, University of Tasmania, Hobart, Tasmania 7001, Australia; dima.kamenetsky@utas.edu.au
4Geophysical Laboratory, Carnegie Institution for Science, 5251 Broad Branch Road NW, Washington DC 20015, USA; mrackerson85@gmail.com
5Geological Institute, Russian Academy of Sciences, 7 Pyzhevsky lane, 119017 Moscow, Russia; pokrov@ginras.ru
6A.P. Vinogradov Institute of Geochemistry, Siberian Branch of the Russian Academy of Sciences, 1a Favorkogo street, 664033 Irkutsk, Russia; vlad@igc.irk.ru
7Sobolev Institute of Geology and Mineralogy, Siberian Branch of the Russian Academy of Sciences, Koptyuga ave. 3, Novosibirsk 630090, Russia; manunex2006@yandex.ru ; klitasov@igm.nsc.ru ; zed@igm.nsc.ru
8Novosibirsk State University, Pirogova st. 2, Novosibirsk 630090, Russia
9Diamond and Precious Metal Geology Institute of the Siberian Branch of the Russian Academy of Sciences, Lenina ave. 39, Yakutsk, 677007, Russia
This webpage is based on Ivanov, A. V., S. B. Mukasa, V. S. Kamenetsky, M. Ackerson, E. I. Demonterova, B. G. Pokrovsky, N. V. Vladykin, M. V. Kolesnichenko, K. D. Litasov, and D. A. Zedgenizov Volatile concentrations in olivine-hosted melt inclusions from meimechite and melanephelinite lavas of the Siberian Traps Large Igneous Province: Evidence for flux-related high-Ti, high-Mg magmatism, Chem. Geol., in press 2018, doi:https://doi.org/10.1016/j.chemgeo.2018.03.011.
Introduction
The origin of high-Mg melts such as picrites, boninites, komatiites and meimechites remains a controversial topic in igneous petrology, with both decompressing high-temperature plumes (e.g., Arndt et al., 1998a, 1998b; Sobolev et al., 1991, 2009) and water-fluxed melting (e.g., Fiorentini et al., 2008; Gurenko & Kamenetsky, 2011) serving as contending models. Sometimes the same rock types are attributed to one or another model (e.g., Arndt, 2003; Parman & Grove, 2005; Sobolev et al., 2016). In a paper recently accepted for publication by Chemical Geology (Ivanov et al., 2018) we address this question by determining H2O, CO2, F, Cl and S concentrations in olivine-hosted melt inclusions from high-Mg volcanic rocks in the Siberian Traps Large Igneous Province. These bracket the main pulse of volcanism at about 252-250 Ma and can be classified as melanephelinites and meimechites. Meimechites are classified by International Union of Geological Sciences as rocks with MgO > 18 wt.% (Le Bas, 2000) and were previously considered as being crystallized from hottest melts in Phanerozoic Earth. In our study (Ivanov et al., 2018) we challenge this point of view.
Results
In order to assess the role of water and temperature in the generation of meimechites we conducted a number of experiments with homogenization of Cr-spinel- and olivine-hosted melt inclusions. These were homogenized at 1400°C at ambient pressure and 1500°C at 5-6 kbar, respectively. Cr-spinel-hosted melt inclusions (sample M9a) were used to determine the MgO concentration of primary melts, which turned out to be ~15.5-17.6 wt.%, less than estimated on olivine-hosted melt inclusions in previous studies (Sobolev et al., 1991; 2009b). Olivine-hosted melt inclusions (samples 1658, 1792, 1656) were used to determine volatile element concentrations by SIMS. When corrected to the primary MgO, the volatiles have a wide range of concentrations with the highest values up to 3.88 wt.% H2O, 1477 ppm CO2, 2490 ppm S, 4214 ppm F and 2.08 wt.% Cl. These are the highest volatile concentrations ever reported for meimechites except for S.
There is a good correlation between H2O and CO2 for meimechite olivine-hosted melt inclusions (Figure 1). This correlation may be either due to degassing of melts during their ascent to the surface or mixing between degassed and less-degassed melts. Irrespective of the cause of this correlation, the initial concentrations of H2O and CO2 could be significantly higher than the highest values measured. Thus, even the highest concentrations in meimechites may be viewed as minimum estimates for the original volatile concentrations in the melt.
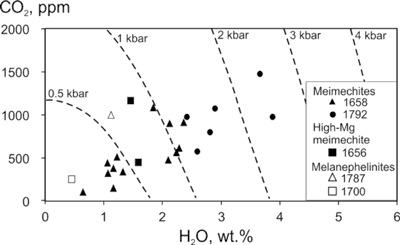
Figure 1: CO2 vs H2O concentrations in olivine-hosted melt inclusions. Isobars at which the melt inclusions were trapped by host olivines are calculated at 1500°C and 41 wt% SiO2 in melt using VOLATILECALC (Newman & Lowenstern, 2002).
Figure 2 shows variations in H2O relative to F, S and Cl in olivine-hosted melt inclusions. There is an obvious correlation between H2O and F. The highest F concentrations in MgO-corrected melt inclusions exceed 0.4 wt.%. Sulphur concentrations are relatively high (500-2300 ppm) and do not correlate with H2O concentrations. Chlorine shows a complex pattern relative to H2O, though the two volatiles seem to be correlated for a majority of the melt inclusions. However, two melt inclusions have extremely high Cl concentrations at relatively low H2O concentrations. High Cl concentrations are obtained by two methods independently – EMP and SIMS (see Ivanov et al., 2018 for details). Some melt inclusions are characterized by low Cl concentrations in awide range of H2O concentrations. There is no correlation between forsterite number of host olivine and water concentrations in melt inclusions (Figure 3).
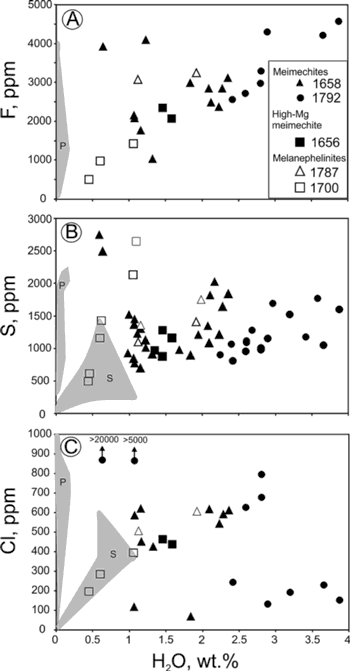
Figure 2: F, S and Cl vs H2O concentrations in olivine-hosted melt inclusions. Results from previous studies are shown by grey fields: clinopyroxene-hosted melt inclusions from melanephelinites (P; Panina & Usoltseva, 2008; Panina & Motorina, 2013), and olivine-hosted melt inclusions from meimechites (S; Sobolev et al., 2009b). Large variations of F, S and Cl at low H2O concentrations in clinopyroxene-hosted melt inclusions are likely due to water loss either due to MI homogenization at 1 atm experiment in Panina & Usoltseva (2008) and Panina & Motorina (2013) or due to water loss in nature from melt inclusions located in clinopyroxenes.
Water and elements of similar incompatibility (K, La, Ce, Pr, Sr, Pb) are expected to correlate if the melts are derived from a single mantle source by simple fractional melting (e.g., Kamenetsky & Eggins, 2012). However, no such correlation exists in subduction-related magmas owing to the variable addition of the subducted component to different domains within the mantle wedge. We find no correlations between water concentrations and, for example, potassium concentrations in olivine-hosted melt inclusions from our study, which suggests that H2O concentrations are not due to difference in degree of partial melting. Decoupling between H2O and K, La, Ce, Pr, Sr, Pb cannot be explained by diffusive over-hydration as suggested for some regions, e.g., Icelandic olivine-hosted melt inclusions (Hartley et al., 2015), because there is correlation between H2O and F for most melt inclusions, or between H2O and Cl for a subset of melt inclusions (Figure 2). In other words, if water was incorporated into melt inclusions because of water diffusion through host-olivine, F and partially Cl should also be incorporated into melt inclusions by the same diffusive processes. This is unrealistic because hydrogen diffusion in olivine is order of magnitude more rapid. Thus, decoupling between H2O and trace elements of similar compatibility suggests fluxing by H2O (and other volatiles).
To understand the source of water and other volatiles we analyzed trace elements and Sr-Nd isotopes in host rocks. Obtained isotope values, especially for meimechites, show that they were primary mantle-derived melts uncontaminated by crust on their passage to the surface (Figure 4). Thus, water and other volatiles are a primary mantle feature.
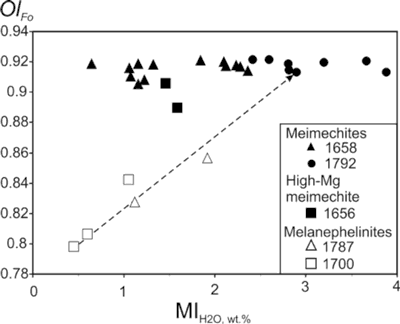
Figure 3: H2O concentrations in melt inclusions relative to forsterite (Fo) number of host olivine. Arrow continues the trend for melanephilinites to Fo of 0.91.
Melanephelinite olivine-hosted melt inclusions (samples 1787 and 1700; Figure 4) show lower concentrations of all volatiles (Figures 1 & 2). However, their host olivines are more fractionated. There is clear positive correlation between the Fo number of host olivine of melanephelinites and water content in their melt inclusions (Figure 3). Continuing the trend toward Fo=0.91 suggests that primary melanephelinite melt could have more than 3 wt.% H2O, similar to the highest H2O values in some meimechite melt inclusions.
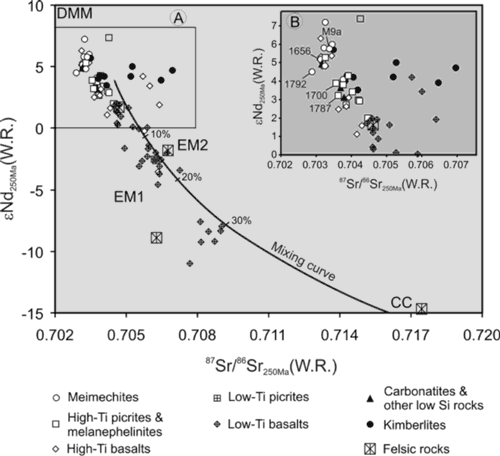
Figure 4: Sr-Nd isotope diagram for all rock types from the Siberian Traps Large Igneous Province (A). Insert (B) is an enlarged part of the diagram showing the positions of samples analyzed in this study, labelled with their sample numbers. For data see references in Ivanov et al. (2018). The bold curve is a hypothetical mixing curve between mantle and continental crust (CC) sources reproduced from Arndt et al. (2003). DMM, EM1 and EM2: depleted mid-oceanic ridge basalt mantle, enriched mantle type 1 and enriched mantle type 2 (after Stracke et al., 2005).
We suggest that meimechites originated in an initially depleted portion of the lithospheric mantle (to satisfy their depleted Sr-Nd-isotopic features), which was fluxed by COH or CHN fluid. Water appears in front of the COH or CHN fluid due to redox reaction and leads to abrupt decrease of the solidus and concomitant flux melting (Taylor & Green, 1986; 1987; Foley, 1988; 2011; Litasov et al., 2014; Sokol et al., 2017). We suggest that the fluids were derived from stagnant slabs, which were likely present beneath Permian-Triassic Siberia (Figure 5). The same stagnant slabs provided hydrous fluids and were also the origin of the low-Ti, low-Mg melts of the Siberian Traps Large Igneous Province (Ivanov & Litasov, 2014). The same stagnant slabs provided eclogitic material, which was the source of earlier high-Ti, high-Mg melts (e.g., the Gudchikhinsky picrites; Ivanov, 2007; Sobolev et al., 2009a). Thus, various types of melt in the Siberian Traps Large Igneous Province were produced by different mechanisms but all were related to continuing subduction beneath this part of Pangea (Figure 5). Alternatively, a low mantle plume could reactivate fluids in the mantle transition zone as, for example, was suggested for the 2.7-Ga Abitibi komatiites (Sobolev et al., 2016). However, no high temperature mantle plume is required to explain the origin of the meimechites (see Discussion below).
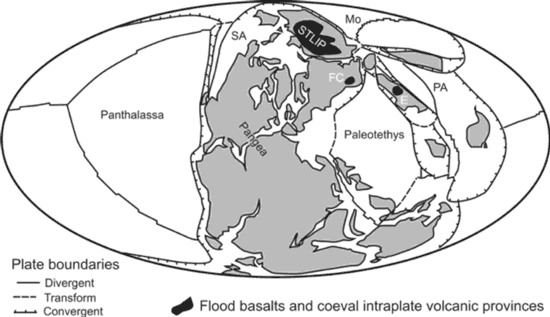
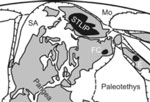
Figure 5: Paleogeographic reconstructions for 270 Ma (Domeier & Torvik, 2014), some ~20 Ma before the main phase of Siberian Traps Large Igneous Province (STLIP) volcanism. Other flood basalt and intracontinental volcanic provinces are Emeishan (E, ca. 260 Ma) and Fore-Caucasus (FC, ca. 230 Ma). Other abbreviations: Mo – Mongol-Okhotsk Ocean; SA – Slide Mountain–Angayucham Ocean; PA—Paleo-Asian Ocean. According to Ivanov et al. (2008), Gladkochub et al. (2010), Ivanov & Litasov (2014), Ivanov (2015) and Wang et al. (2016) subduction of the Mongol-Okhotsk oceanic slab beneath Siberia played a critical role in generation of the STLIP with or without influence from a lower-mantle plume.
Discussion
Irrespective of the source of the volatiles, high concentrations of water and other volatiles in meimechites have important implications for the origin and petrology of high-Mg melts in general. From experimental studies of melting various fertile and depleted lherzolite compositions in wet conditions at 1-2 GPa, it is known that water and pressure play opposite roles in the compositions of the derived melts. Addition of water shifts the melts towards quartz-normative compositions, whereas increasing pressure drives the melts to olivine-normative compositions (Mitchell & Grove, 2015). To match this general rule with the primary meimechite melt composition found in this study (i.e., silica undersaturated, high in magnesium and rich in water) the pressure for melt generation was probably 2 GPa or higher. This would allow pressure to counteract the role of water and drive the melt toward high-Mg compositions. For example, Elkins-Tanton et al. (2007) derived 5.5 GPa (~180 km depth) from their experimental study for the pressure of meimechite origination.
High bulk-rock Mg concentrations and high Fo number for olivine grains from meimechite samples were long used as evidence for the primary magmas of these rocks having a high melting temperature (e.g., Sobolev et al., 1991). For a meimechite sample suite similar to ours, Sobolev et al. (2009b) calculated potential temperature of 1650°C, and therefore invoked a hot plume source. Affirming that calculation with experiments, Elkins-Tanton et al. (2007) showed that meimechite melts with ~1 wt.% H2O are produced at 1700°C. However, in our study we show that MgO concentrations in the primary meimechite melts were < 18 wt.%. Strictly speaking, there were no meimechite melts–the primary melt was melanephelinitic in composition–and the high MgO in all meimechite rocks is due to olivine accumulation (Ivanov et al., 2018). Thus, calculations based on previous estimates of MgO in meimechites (> 22 wt.%) leads to erroneously high temperatures. Second, high concentrations of H2O and other volatiles, as recorded in our study, may decrease the temperature of melting considerably. For example, Medard & Grove (2008) have shown that addition of H2O depresses the melting temperature irrespective of pressure according to the following empirically derived equation:
ΔT (°C) = 40.4(H2Omelt, %) – 2.97(H2Omelt, %)2 + 0.0761(H2Omelt, %)3.
Thus, using 3.88 wt.% as the minimum estimate of H2O in the primitive meimechite melt reduces the temperature by at least 116°C. This is only a minimum estimate in melting temperature reduction because significant amounts of F and CO2 in the system were also a factor in the actual total volatile inventory.
Numerous experiments show that CO2 depresses the melting point of mantle sources because of increased entropy due to the low solubility in silicate magmas (e.g., Gudfinnsson & Presnall, 2005; Dasgupta et al., 2007). Experiments with F are rare. For example, Brey et al. (2009) studied experimentally carbonated lherzolite + H2O and carbonated wherlite + F and found that F has about the same effect as H2O in depressing the melting temperature of the source region. In both cases, the solidus of carbonated sources either with H2O or F is < 1100°C at 6 GPa. Hence a depression of melting temperature of up to 600°C can be expected in comparison to the experiments by Elkins-Tanton et al. (2007). Another important observation from the experiments by Brey et al. (2009) is that addition of F to the system shifts the melt towards higher MgO and CaO concentrations and slightly poorer Al2O3 and SiO2 concentrations. This is a primary characteristic of meimechite melts.
Summary
-
In our study we estimated primary meimechite melt compositions from homogenization of Cr-spinel-hosted melt-inclusions at 1400°C and 1 atm and showed that the primary meimechite melt has ~15.5-17.6 wt.% MgO on a volatile-free basis. This is significantly lower compared to results obtained previously using homogenized olivine-hosted melt-inclusions.
-
We measured high concentrations of volatile components (H2O, CO2, S, F, Cl) in the olivine-hosted melt inclusions from meimechites. They are the highest (except for S) ever reported for meimechites. This resulted from the serendipitous discovery of the least degassed sample and use of a homogenization technique at pressure which prevented loss of H2O during the MI homogenization procedure.
-
Lower MgO and high volatile concentrations in primary meimechite melts have the important implication that previously considered high-temperature melts were, on the contrary, generated at modest temperatures in a volatile-rich environment.
Acknowledgements
This study was made possible by financial support from the Russian Science Foundation project no. 16-17-10068 to AVI and EID and National Science Foundation grant OPP-1025513 to SBM. VSK acknowledges Australian Research Council (discovery grants DP1092823 and DP130100257). AVI acknowledges a Fulbright Scholarship that supported a research visit to the USA. FTIR analyses were funded by the Russian Foundation for Basic Research project no. 16-35-00317 and projects of the Ministry of Education and Science of the Russian Federation nos. 14.B25.31.0032 and 14.Y26.31.0018 to MVK, KDL and DAZ.
References
-
Arndt, N., 2003. Komatiites, kimberlites, and boninites. J. Geophys. Res., 108, B6, 2293, doi:10.1029/2002JB002157.
-
Arndt, N., Chauvel, C., Czamanske, G., and Fedorenko, V., 1998a. Two mantle sources, two plumbing systems: Tholeiitic and alkaline magmatism of the Maymecha River basin, Siberian flood volcanic province. Cont. Min. Pet., 133, 297–313, doi: 10.1007/s004100050453.
-
Arndt, N.T., Czamanske, G.K., Walker, R.J., Chauvel, C., and Fedorenko, V.A., 2003. Geochemistry and origin of the intrusive hosts of the Noril'sk-Talnakh Cu-Ni-PGE sulfide deposits. Economic Geology, 98, 495-515.
-
Arndt, N.T., Ginibre, C., Chauvel, C., Albarede, F., Cheadle, M., Herzberg, C., Jenner, G., and Lahaye, Y., 1998b. Were komatiites wet? Geology, 26, 739-742, doi: 10.1130/0091-7613(1998)026<0739:WKW>2.3.CO;2.
-
Brey, G.P., Bulatov, V.K., and Girnis, A.V., 2009. Influence of water and fluorine on melting of carbonated peridotite at 6 and 10 GPa. Lithos, 112, 249–259, doi:10.1016/j.lithos.2009.04.037.
-
Dasgupta, R., Hirschmann, M.M., and Smith, N.D., 2007. Partial melting experiments of peridotite + CO2 at 3 GPa and genesis of alkalic ocean island basalts. J. Pet., 48, 2093-2124, doi: 10.1093/petrology/egm053.
-
Elkins-Tanton, L.T., Draper, D.S., Agee, C.B., Jewell, J., Thorpe, A., and Hess, P.C., 2007. The last lavas erupted during the main phase of the Siberia flood volcanic province: results from experimental petrology. Cont. Min. Pet., 153, 191-209, doi: 10.1007/s00410-006-0140-1.
-
Fiorentini, M.L., Beresford, S.W., Deloule, E., Hanski, E., Stone, W.E., and Pearson, N.J., 2008. The role of mantle-derived volatiles in the petrogenesis of Palaeoproterozoic ferropicrites in the Pechenga Greenstone Belt, northwestern Russia: Insights from in-situ microbeam and nanobeam analysis of hydromagmatic amphibole. Earth Planet. Sci. Lett., 268, 2-14, doi: 10.1016/j.epsl.2007.12.018.
-
Foley, S.F., 1988. The genesis of continental alkaline magmas – An interpretation in terms of redox melting. J. Pet., Special Lithosphere Issue, 139-161.
-
Foley, S.F., 2011. A reappraisal of redox melting in the Earth’s mantle as a function of tectonic setting and time. J. Pet., 52, 1363-1391, doi: 10.1093/petrology/egq061.
-
Gladkochub, D.P., Donskaya T.V., Ivanov, A.V., Ernst, R., Mazukabzov, A.M., Pisarevsky, S.A., and Ukhova, N.A., 2010. Phanerozoic mafic magmatism in the southern Siberian craton: geodynamic implication. Russian Geology and Geophysics, 51, 952-964, doi: 10.1016/j.rgg.2010.08.005.
-
Gudfinnsson, G.H., and Presnall, D.C., 2005. Continuous Gradations among Primary Carbonatitic, Kimberlitic, Melilititic, Basaltic, Picritic, and Komatiitic Melts in Equilibrium with Garnet Lherzolite at 3–8 GPa. J. Pet., 46, 1645-1659, doi: 10.1093/petrology/egi029.
-
Gurenko, A.A., and Kamenetsky, V.S., 2011. Boron isotopic composition of olivine-hosted melt inclusions from Gorgona komatiites, Colombia: New evidence supporting wet komatiite origin. Earth Planet. Sci. Lett., 312, 201-212, doi: 10.1016/j.epsl.2011.09.033.
-
Domeier, M., and Torvik, T.H., 2014. Plate tectonics in the late Paleozoic: Geoscience Frontiers, 5, 303-350, doi:10.1016/j.gsf.2014.01.002.
-
Hartley, M.E., Neave, D.A., Maclennan J., Edmonds, M., and Thordarson, T., 2015. Diffusion over-hydration of olivine-hosted melt inclusions. Earth Planet. Sci. Lett., 425, 168-178, doi: 10.1016/j.epsl.2015.06.008.
-
Ivanov, A.V., 2007. Evaluation of different models for the origin of the Siberian Traps, in Foulger, G.R., and Jurdy, D.M., eds., Plates, Plumes, and Planetary Processes: Geological Society of America Special Paper 430, 669-689, doi: 10.1130/2007.2430(31).
-
Ivanov, A.V., Demonterova, E.I., Rasskazov, S.V., and Yasnygina, T.A., 2008. Low-Ti melts from the southeastern Siberian Traps Large Igneous Province: Evidence for a water-rich mantle source? Journal of Earth System Science, 117, 1-21, doi: 10.1007/s12040-008-0008-z.
-
Ivanov, A.V., and Litasov, K.D., 2014. The deep water cycle and flood basalt volcanism. Int. Geol. Rev., 56, 1–14, doi: 10.1080/00206814.2013.817567.
-
Ivanov, A.V., Mukasa, S.B., Kamenetsky, V.S., Ackerson, M., Demonterova, E.I., Pokrovsky, B.G., Vladykin, N.V., Kolesnichenko, M.V., Litasov K.D., and Zedgenizov D.A., 2018. Volatile concentrations in olivine-hosted melt inclusions from meimechite and melanephelinite lavas of the Siberian Traps Large Igneous Province: Evidence for flux-related high-Ti, hij-Mg magmatism. Chem. Geol., in press, 2018, doi: 10.1016/j.chemgeo.2018.03.011.
-
Kamenetsky, V.S., and Eggins, S.M., 2012. Systematics of metals, metalloids, and volatiles in MORB melts: Effects of partial melting, crystal fractionation and degassing (a case study of Macquarie Island glasses). Chem. Geol., 302-303, 76-86, doi: 10.1016/j.chemgeo.2011.04.008.
-
Le Bas, M.J., 2000. IUGS recalculation of the high-Mg and picritic volcanic rocks. J. Pet., 41, 1467-1470, doi: 10.1093/petrology/41.10.1467.
-
Litasov, K.D., Shatskiy, A., Ohtani, E., 2014. Melting and subsolidus phase relations in peridotite and eclogite systems with reduced C-O-H fluid at 3-16 GPa, Earth Planet. Sci. Lett., 391, 87-99.
-
Medard, E., and Grove, T.L., 2008. The effect of H2O on the liquidus of basaltic melts: experiments and thermodynamic models. Cont. Min. Pet., 155, 417-432, doi: 10.1007/s00410-007-0250-4.
-
Mitchell, A.L., and Grove, T.L., 2015. Melting the hydrous, subarc mantle: the origin of primitive andesites. Cont. Min. Pet., 170, 13, doi: 10.1007/s00410-015-1161-4.
-
Newman, S., and Lowenstern, J.B., 2002. VolatileCalc: a silicate melt-H2O-CO2 solution model written in Visual Basic for excel. Computers & Geosciences, 28, 597–604, doi: 10.1016/S0098-3004(01)00081-4.
-
Panina, L.I., and Motorina, I.V., 2013. Meimechites, porphyritic alkaline picrites, and melanephelinites, of Siberia: conditions of crystallization, parental magmas, and sources. Geochemistry International, 51, 109-128, doi:10.1134/S0016702913020080.
-
Panina, L.I., and Usoltseva, L.M., 2008. Alkaline-ultrabasic mantle-derived magmas, their sources, and crystallization features: Data of melt inclusion studies. Lithos, 103, 431-444, doi: 10.1016/j.lithos.2007.10.009.
-
Parman, S.W., and Grove, T.L., 2005. Komatiites in plume debate, in Foulger, G.R., Natland, J.H., Presnall, D.C., and Anderson, D.L., eds., Plates, Plumes, and Paradigms: Geological Society of America Special Paper 388, 249-256, doi: 10.1130/0-8137-2388-4.249.
-
Sobolev, A.V., Asafov, E.V., Gurenko, A.A., Arndt, N.T., Batanova, V.G., Portnyagin, M.V., Garbe-Schonberg, D., and Krasheninnikov, S.P., 2016. Komatiites reveal a hydrous Archaean deep-mantle reservoir. Nature, 531, 628-632, doi: 10.1038/nature17152.
-
Sobolev, A.V., Kamenetsky, V.S., Kononkova, N.N., 1991. New data on petrology of Siberian meimechites. Geokhimiya, No. 8, 1084-1095.
-
Sobolev, A.V., Kuzmin, D.V., and Krivolutskaya, N.A., 2009a. Petrology of the parental melts and mantle sources of Siberian Trap magmatism. Petrology, 17, 253-286, doi: 10.1134/S0869591109030047.
-
Sobolev, A.V., Sobolev, S.V., Kuzmin, D.V., Malitch, K.N., and Petrunin, A.G., 2009b. Siberian meimechites: origin and relation to flood basalts and kimberlites. Russian Geology and Geophysics, 50, 999-1033, doi: 10.1016/j.rgg.200.
-
Sokol A.G., Tomilenko, A.A., Bul'bak, T.A., Palyanova, G.A., Sokol, I.A., and Palyanov, Y.N., 2017. Carbon and Nitrogen Speciation in N-poor C-O-H-N Fluids at 6.3 GPa and 1100-1400 degrees C. Scientific Reports, 7, art. no. 706, doi: 10.1038/s41598-017-00679-7.
-
Stracke, A., Hofmann, A.W., and Hart, S.R., 2005. FOZO, HIMU, and the rest of the mantle zoo. Geochem. Geophys. Geosys., 6, Q05007.
-
Taylor, W.R., and Green, D.H., 1986. The role of reduced C-O-H fluids in mantle partial melting. in Jaques, A.L., ed., Kimberlites and related rocks. Volume 1. Their composition, occurrence, origin and emplacement. Proceedings of the fourth international kimberlite conference: Geological Society of Australia Special Publication 14, 592-602.
-
Taylor, W.R., and Green, D.H., 1987. The petrogenetic role of methane: effect of liquidus phase relations and the solubility mechanism of reduced C-H volatiles. in Mysen, B.O., ed., Magmatic processes: Physicochemical principles: The Geological Society (London), Special Publication 1, 121-138.
-
Wang, X.-C., Wilde, S.A., and Pang, C.-J., 2016. Origin of arc-like continental basalts: Implications for deep-Earth fluid cycling and tectonic discrimination. Lithos, doi:10.1016/j.lithos.2015.12.014.
last updated 22nd
March, 2018 |
